The following article was sent to me by subscriber Suzanne Crookshanks on Lactoferrin. It was written by several authors in Poland and published May 4, 2022 on PubMed.
The Lactoferrin Phenomenon—A Miracle Molecule
Paweł Kowalczyk,1 Katarzyna Kaczyńska,2 Patrycja Kleczkowska,3,4,* Iwona Bukowska-Ośko,5 Karol Kramkowski,6 and Dorota Sulejczak7,*
I don’t understand the chemistry of “chelation” works in the human body. First of all, here is a definition of “Chelation” from Wikipedia.
Chelation is a type of bonding of ions and the molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom.[1][2] These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity.
The word chelation is derived from Greek χηλή, chēlē, meaning "claw"; the ligands lie around the central atom like the claws of a crab. The term chelate was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or chele (Greek) of the crab or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings."[3]
This sounds very familiar to me to a process I once used in the steel industry while desulfurizing liquid steel. Sulfur is a very undesirable element in steel, because during the casting process, the sulfur molecules tend to bind together and form long chains, leading to a greatly increased risk of cracking in the finished steel product.
In order to remove the sulfur, we first had to remove the oxygen from the raw steel from the furnace by adding aluminum to the steel. The aluminum binds with the oxygen and floats to the top of the steel in the form of aluminim oxide as “slag.” Next, to remove the sulfur, you stir in lime, or calcium oxide, which binds to the sulfur and floats to the top as Ca2SO4. You then add the alloys, adjust temperature, and cast the steel out from the bottom of the ladle, and discard the slag.
Lactoferrin is a very interesting topic to me, one that Suzanne and I have discussed frequently. It is something she takes to help with her cancer, which is essentially in remission the last I spoke with her.
I first became aware of it in some discussions of a test to cure cancer with Ivermectin. Stefan Hartmann, of Iron Direct Primary Care, suggested also using Lactoferrin.
Stefan Hartmann, PA updates Ivermectin and Lactoferrin Volunteer Trials on Cancer and Enlarged Prostate
Stefan Hartmann of Iron Direct Primary Care in Melbourne, Florida led a videoconference presentation Thursday on the progress of volunteer study treating cancer and enlarged prostate with Ivermectin and Lactoferrin. For more details on the study please join their Viber Group (details below).
Lactoferrin works to kill both cancer cells and parasites that feed on the cancer cells by stealing the iron from them. It is a natural compound found in milk, although the Pasteurization process destroys it, so people cannot get the benefit of it. I suspect that there are many beneficial molecules in milk which are destroyed during the Pasteurization process.
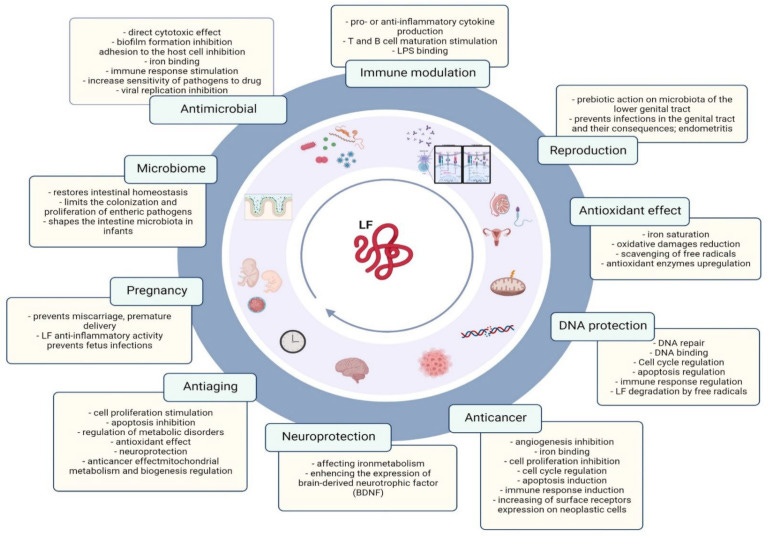
Getting to the referenced article, The Lactoferrin Phenomenon—A Miracle Molecule, I was somewhat surprised to see that the compound may bind to more more than Iron alone.
First the Abstract.
Numerous harmful factors that affect the human body from birth to old age cause many disturbances, e.g., in the structure of the genome, inducing cell apoptosis and their degeneration, which leads to the development of many diseases, including cancer. Among the factors leading to pathological processes, microbes, viruses, gene dysregulation and immune system disorders have been described. The function of a protective agent may be played by lactoferrin as a “miracle molecule”, an endogenous protein with a number of favorable antimicrobial, antiviral, antioxidant, immunostimulatory and binding DNA properties. The purpose of this article is to present the broad spectrum of properties and the role that lactoferrin plays in protecting human cells at all stages of life.
The authors describe the iron chelation process which steals iron from pathogens below. This is part of the anticancer effect. Once Lactoferrin binds to the Iron, I don’t know what happens to it after that.
LF is a protein with multifaceted effects on the body, not all mechanisms of which have been thoroughly investigated yet, which is why it is referred to as a multipotent protein [16]. Chemically, it is a glycoprotein that, due to its homology of sequence with serum transferrin, is classified as a member of the transferrin family, a protein that can bind to iron ions. Lactoferrin, as the name suggests (lacto + ferrin = milk + iron), is iron binding milk protein, which helps to balance iron levels in the body [11,12,17]. Excess iron can be toxic because it has the ability to donate electrons to oxygen, resulting in the formation of reactive oxygen species (ROS) such as superoxide anions and hydroxyl radicals. LF, thanks to the ability to strongly and reversibly bind iron ions, supports the body in maintaining the homeostasis of this important micronutrient. LF has a high affinity for iron, several hundred times greater than the affinity of transferrin [18], and each lobe of LF can bind to an iron ion [13]. There is a high probability that lactoferrin can also bind copper, zinc and manganese ions [10,19]. In addition, as a result of the chelation process, which reduces the iron overload caused by the accumulation of iron in many organs, leading to free radical generation and dysfunction, the availability of Fe to pathogens that need it for their growth is reduced [20].
As for graphene, they don’t mention it, but they do mention “There is a high probability that lactoferrin can also bind copper, zinc and manganese ions.” Therefore I ask the question if Lactoferrin could also bind to Graphene? And the other question is, if Lactoferring does bind to Graphen, would it be removed from the body afterwards?
It clearly needs more study, but I thought it would bring it to your attention. In any case I don’t have any reservations recommending that people drink natural milk.
Charles Wright





Thanks I've been looking at ways to remove excess iron (Haemochromatosis) as I think the excess iron is helping the self assembly polymer
Nanoparticular graphene oxide has been studied as a delivery mechanism for lactoferrin in the treatment of cancer. So it appears lactoferrin must bind to GO. As you pointed out, though, it is unclear if lactoferrin removes GO from the body. Of course, the barbaric researchers didn’t address what ultimately happens to GO once it is injected into the body (or if the authors did, they didn’t bother to mention it in this discussion).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10224286/#B29-pharmaceutics-15-01362