Remdesivir was used in New York and China to create deaths and simulate the spread of a deadly virus.
I have reached a conclusion that Remdesivir was used in Wuhan and New York to kill COVID patients in order to simulate the spread of a deadly virus. I am not suggesting that viruses or bioweapons do not exist, or that they were not a factor in the COVID-19 Pandemic. I am saying that whatever virus or bioweapon that existed was not responsible for the high death rates - Remdesivir was. In order to create a larger scare in the public, the powers that be conspired to kill people with Remdesivir in order to create the impression of a rapidly spreading deadly virus.
This is not a simple conclusion to prove in one sitting. In this article I will attempt to prove one major aspect that is central to my conclusion - that Northwell Health in New York deliberately killed patients using Remdesivir in a study that was funded to be a “Famotidine” study, and then blamed the Remdesivir deaths on COVID.
The ASPR contracted Alchem Laboratories in Florida for what they described as “Famotidine” trials. Alchem in turn subcontracted the study to Northwell in New York. Famotidine is the active ingredient of Pepcid AC. While Famotodine was used in the Northwell trials, it was also used with Remdesivir. Remdesivir was used in both the test and placebo groups receiving Famotidine. Northwell reported the death statistics as "Famotidine” and “not Famotidine,” and did not disclose in any understandable way that the deaths in both groups were caused by Remdesivir, not COVID.
The intentional deception at Northwell, using Remdesivir where the public thought it was Famotidine, suggests that the same strategy to use Famotidine and Remdesivir was used in the Wuhan hospital system. Michael Callahan “supervised” treatments at Wuhan, according to Robert Malone. Callahan then likely brought data on Famotidine/Remdesivir death rates in Wuhan back to the United States. In one group using Famotidine, the death rates were lower than another group using Omeprazole. The cause of death was assumed to be COVID, however. These death rates are far in excess of what can reasonably expected from COVID, suggesting that Remdesivir was also used in Wuhan and was the primary cause of death there.
Remdesivir was definitely used in China. Gilead Sciences, the patent holder of Remdesivir, began small trials of Remdesivir in China in early February. Anthony Fauci sent an email confirming shipment of Remdesivir to China. Some shipments of Remdesivir may have had a “compassionate use” basis. China copied Remdesivir and mass produced it.
This article will compare three studies using Famotidine to treat COVID-19: 1. Michael Callahan’s trial in Wuhan, China; 2. Northwell Health’s trials at their hospital system in New York; 3. and Robert Malone’s small trial in Wisconsin.
The comparison is a statistical proof of sorts. Northwell’s trials had a high death rate, and used Famotidine and Remdesivir. Callahan’s Wuhan trials had high death rates associated with Famotidine and Omeprazole. Malone’s small Famotidine trial in Wisconsin had a 100% success rate.
This suggests to me that Remdesivir was used in Wuhan in the same manner it was used in New York, to kill patients and blame the deaths on COVID.
In the background of all of the death rates that will be reported here, it is very important to understand that numerous doctors treated thousands of COVID patients with 100% success rates. That means that all the reported high death rates were caused by something other than COVID.
There will be a chronology section below the main article with more detailed information in areas.
Trial 1: Wuhan, Famotidine, Omeprazole, and ?
First, I have to establish that treatments administered in Wuhan constituted a trial of sorts. It seems very likely that Michael Callahan was involved in administering drugs in Wuhan, China. What limited reporting there is on Michael Callahan frames him as an observer of treatments in China, not a supervisor. A comment made by Robert Malone, however, described Michael Callahan as supervising treatments.
In any case, whether you call it a trial or not, the facts as reported are that there was a high “COVID” death rate reported in the presence of Famotidine and Omeprazole. From Science, April 26, 2020.
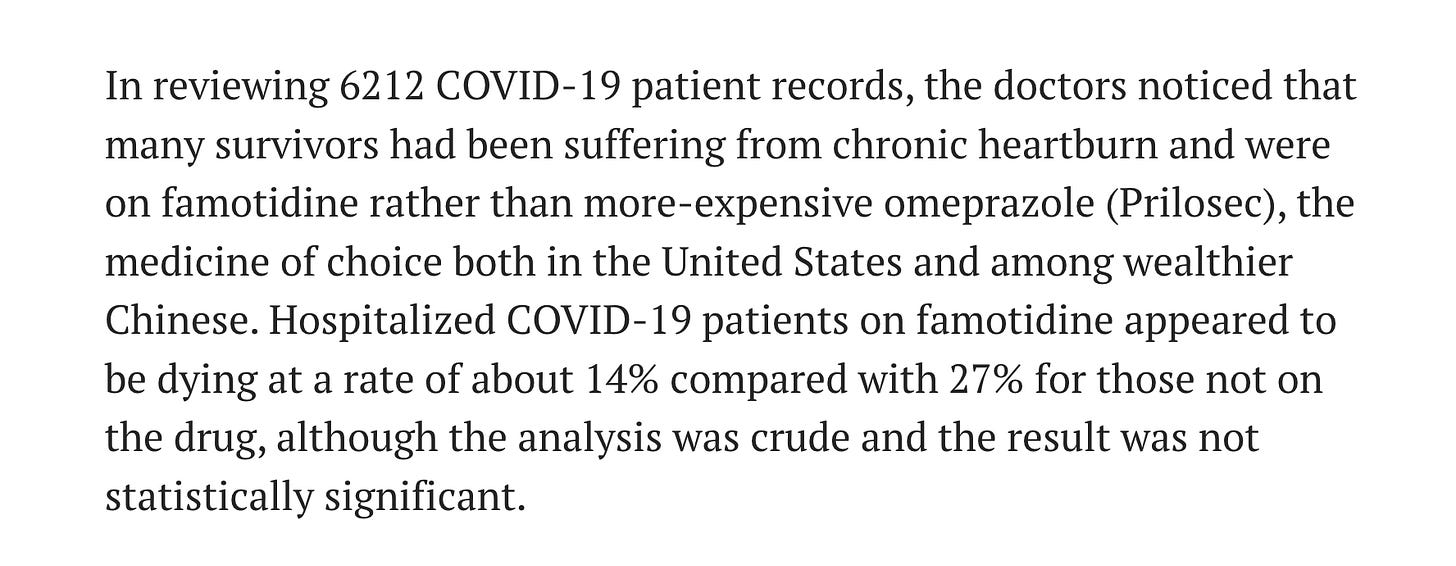
Michael Callahan gathered 6,212 patient records in Wuhan. The exact dates and other details are unknown. He was reported to be in China from at least sometime in November and well into January before returning to the United States. I assume that Callahan gathered statistics on COVID treatments in the early stages of the Pandemic in China, throughout January, before it spread to the United States.
600 of the 6,212 COVID patients in WUhan were using Famotidine (Pepcid AC). An unknown number were using Omeprazole (Prilosec). AP News, July 23, 2020.
14% of the 600 COVID patients in Wuhan who were using Famotidine died. 27% of an unknown number of COVID patients who were using Omeprazole died.
It is important to understand that here was no reason or way that people died at rates this high from “COVID” whether or not Famotidine or Omeprazole was used. Something else was killing the patients - not COVID, not Famotidine, and not Omeprazole.
Trial 2: Northwell Health, Famotidine, Hydroxychloroquine, and Remdesivir.
First, some brief background. Michael Callahan came back to the United States at some unreported date and signed a contract with Robert Kadlec “a few days” after January 28, 2020. Gilead began a small trial of Remdesivir in China in early February. Fauci’s NIAID began trials of Remdesivir on February 25, 2020, and noted that clinical trials of Remdesivir were also underway in China.
In late February, Robert Malone said that he contracted COVID. Malone said that took Famotidine as an antidote because he had identified Famotidine as a potential COVID treatment from computer screening models at Alchem Laboratories. Malone said the treatment cured his COVID and announced his discovery of Famotidine as a cure for COVID on his LinkedIn page.
On March 20, 2020, Robert Kadlec of ASPOR contacted Kevin Tracey at Northwell to request a review of a clinical trial that Northwell was working on with Alchem. Kadlec directed Tracey to ensure that Michael Callahan be involved in the study. I believe that Kadlec wanted to make sure that Callahan was involved because Callahan knew how to create deaths from Remdesivir and ventilators based on his experience in Wuhan. Robert Malone noted the ventilator deaths:
“Based on this timeline and history, as well as my own direct personal communication with Dr. Callahan, I strongly suspect that both the gross clinical mismanagement of ventilatory support during the first phase of the outbreak (responsible for up to 30,000 deaths) as well as the stunningly poor management practices of Nursing Home and Extended Care facilities throughout the USA can be directly traced to the influence of Dr. Michael Callahan.”
While I also believe that ventilator were a major factor, along with opioids and denial of nutrition, Malone did not use the word “Remdesivir” in Wuhan in the referenced article. I believe Remdesivir was the primary means of murder in the name of “COVID.”
It is important to note that because Callahan can create deaths with ventilators, he could also create death rates in clinical trials using ventilators to get the clinical results they wanted.
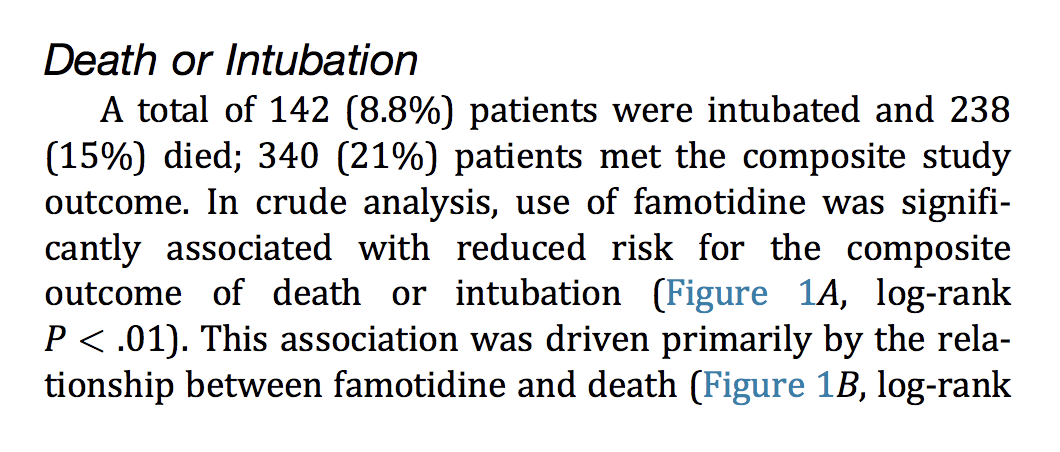
Now on to the “Famotidine” study at Northwell: Famotidine Use Is Associated With Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study
238 of the 1620 patients died. 238 divided by 1620 is 14.69%. That’s where they get the 15% death rate from. They said that a 15% death rate represented “reduced risk for the composite outcome of death or intubation.” This is where the study authors were deceptive to the point of lying. The Famotidine did not prevent “COVID” deaths. Remdesivir and ventilators were killing the patients.
Kevin Tracey, Michael Callahan et al referred to Figures 1A and 1B, below. They represent the groups as “Famotidine” and “no Famotidine.” That’s true enough, but it is a blatant misrepresentation of the nature of the “study” and the cause of death - Remdesivir. The authors of the paper did not mention the word “Remdesivir” once.
The two groups they were comparing were not “Famotidine” and “No Famotidine.” The two groups were Famotidine + Remdesivir, and Placebo + Remdesivir. It’s true to say that one group received Famotidine and the other did not, but they did not report that both groups received Remdesivir.
Where do I get the information from that the study used Remdesivir?
According to clinical trials dot gov, the two groups were Standard of Care + Famotidine, and Standard of Care + Placebo. Remdesivir was the Standard of Care.
Here’s the text where the change in SOC was reported. “On 29 April 2020, the National Institute of Allergy and Infectious Diseases (NIAID) announced that Remdesivir was better than placebo in reducing time to recovery for people hospitalized with advanced COVID-19 and lung involvement. In an earlier study of adult patients admitted to a hospital for severe COVID-19, Remdesivir was not associated with statistically significant clinical benefits. In that study, Remdesivir was not associated with a difference in time to clinical improvement. Although not statistically significant, patients receiving Remdesivir had a numerically faster time to clinical improvement than those receiving placebo among patients with symptom duration of 10 days or less. Remdesivir was stopped early because of higher numbers of adverse events compared to placebo. Because of these studies the FDA stated on 1 May 2020, that it is "reasonable to believe" that known and potential benefits of Remdesivir outweigh its known and potential risks, in some specific populations hospitalized with severe COVID-19. Given the refinement of standard of care to include Remdesivir and no longer hydroxychloroquine, we have edited the study protocol to reflect this new standard of care.”
It’s unclear if the authors included any Hydroxychloroquine statistics in their report.
By not reporting plainly that both the famotidine and placebo groups also received Remdesivir, it is an intentional statistical deception on the part of the study designers and authors. Not only that, they should have been well aware that they were killing patients with Remdesivir needlessly.
Trial 3: Beloit Memorial Hospital, Wisconsin.
This trial was led by Robert Malone.
It is interesting that Malone’s trial was Famotidine/Celecoxib and not Famotidine/Hydroxychloroquine. Malone et al reported a 100% survival rate and good results overall. They did not use ventilators in the study. They did not report the use of a placebo or control group.
The 100% success rate of Famotidine without Remdesivir versus the high death rate of Famotidine with Remdesivir proves to me that Remdesivir was the primary cause of death in Northwell.
OTHER TREATMENTS AND SUCCESS RATES
There are too many doctors to be acknowledged that have had 100% or near 100% survival rates of COVID patients. The methods they have used have varied. The undeniable fact is that the disease has a high survival rate with proper treatment, and proper treatment means not using Remdesivir or mRNA vaccines. Those things are exactly the wrong things to do and are responsible for the deaths. Again, in the early stages of the COVID-19 Pandemic before the mRNA vaccines became available, the only reason people were dying appears to be Remsdesivir protocols. There are too many doctors with 100% success rates not using Remdesivir to ever believe that the clinical trials that had high death rates in both the test and control groups were ever sincere efforts to find a cure.
CHRONOLOGY
A brief chronology of events follows to help the reader and myself understand the timeline of the Pandemic.
OCTOBER, 2019. According to reports from Mike Pompeo and John Ratcliffe, 3 workers at the Wuhan Institute of Virology fell ill sometime in October 2019. Ratcliffe: "What Mike Pompeo and I put out is, you know, people became sick in the lab in October, with symptoms that became entirely consistent with what most people have experienced around the world from COVID-19." It should be noted that in the presence of Michael Callahan, illness of coworkers from infectious diseases is not unprecedented. Callahan:
NOVEMBER, 2019. “Michael Callahan, an infectious disease expert, was working with Chinese colleagues on a longstanding avian flu collaboration (H5N1?) in November when they mentioned the appearance of a strange new virus. Soon, he was jetting off to Singapore to see patients there who presented with symptoms of the same mysterious germ.” Brenden Borrell, National Geographic. (I have seen some speculation that Callahan traveled to Singapore to have the virus sequenced, but like most things surrounding Callahan’s COVID itinerary, it is a mystery).
NOVEMBER, 2019. “US intelligence agencies alerted Israel to the coronavirus outbreak in China already in November, Israeli television reported Thursday. According to Channel 12 news, the US intelligence community became aware of the emerging disease in Wuhan in the second week of that month and drew up a classified document. Information on the disease outbreak was not in the public domain at that stage — and was known only apparently to the Chinese government. US intelligence informed the Trump administration, “which did not deem it of interest,” but the report said the Americans also decided to update two allies with the classified document: NATO and Israel, specifically the IDF. Times of Israel.
December 08, 2020. The WHO reports this date as the first onset of symptoms later called COVID-19.
DECEMBER 11, 2019. DOMANE publication date. Discovery of Medical Countermeasures Against Novel Coronavirus. This is the system that Robert Malone said he used at Alchem to discover Famotidine.
DECEMBER 30, 2019. “On 30 December 2019, three bronchoalveolar lavage samples were collected from a patient with pneumonia of unknown etiology – a surveillance definition established following the SARS outbreak of 2002-2003 – in Wuhan Jinyintan Hospital. Real-time PCR (RT-PCR) assays on these samples were positive for pan-Betacoronavirus. Using Illumina and nanopore sequencing, the whole genome sequences of the virus were acquired. Bioinformatic analyses indicated that the virus had features typical of the coronavirus family and belonged to the Betacoronavirus 2B lineage.” Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)
JANUARY 03, 2020. “Information on the epidemic was notified to WHO on 3 January”. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)
JANUARY 04, 2020: Robert Malone to Joe Rogan: “There's a CIA agent that I've co-published with in the past named Michael Callahan. He was in Wuhan in the fourth quarter of 2019. He called me from Wuhan on January 4. I was currently managing a team that was focused on drug discovery for organophosphate poisoning or (unknown word) nerve agents, for DTRA. Involving high-performing computing and biorobot screening. High end stuff. And he told me Robert you gotta get your team spun up because we've got a problem with this new virus. I worked with him through prior outbreaks.”
JANUARY 07, 2020. WHO: “By Jan 7, 2020, Chinese scientists had isolated a novel coronavirus (CoV) from patients in Wuhan.”
JANUARY 10, 2020. “After isolating the virus from patients, Chinese scientists on Jan. 10 posted online its genetic sequence. Because companies that work with messenger RNA don’t need the virus itself to create a vaccine, just a computer that tells scientists what chemicals to put together and in what order, researchers at Moderna, BioNTech, and other companies got to work.” Stat News.
JANUARY 11, 2020. “I started modeling a key protein protease inhibitor of this virus when the sequence was released on January 11 as the Wuhan Seafood Market virus.” - Robert Malone on the Joe Rogan Show.
JANUARY 17, 2020. Michael Callahan arrives by plane in Nanjing, China, according to Brendan Borrell. Borrell did not report where Callahan traveled from.
JANUARY 28, 2020. 1.28.20. Michael Callahan emails Robert Kadlec, Assistant Secretary for Preparedness and Response of the U.S. Department of Health and Human Services, (ASPR), and told him that the situation in China was worse than the media was reporting.
LATE JANUARY 2020. “In late January 2020 Pompeo asked Yu to investigate the possibility of a leak from the WIV. Yu’s report, dated 26 April 2020, found there was ‘no direct, smoking-gun evidence’ but ‘persuasive circumstantial evidence’ for a ‘possible leak’. Guardian.
"Within days after January 28, 2020." Robert Kadlec hires Michael Callahan. Kadlec had known Callahan for around 20 years. Callahan is required to be on call at all times and within short distance of an airport. Callahan is not allowed to speak to media. Callahan is told he does not represent the United States. From Rolling Stone: "Within days (of a January 28, 2020 email to Robert Kadlec), Kadlec had signed Callahan up for a six-month stint helping ASPR to provide virus intelligence by way of his Chinese connections and advise on how to respond to virus inside the United States. “His physical location and meetings will be determined by the ASPR,” read his statement of work. “He will be expected to respond on ICS [Incident Command System] timelines (24/7 mobile access: 2hr to airport; autonomous resources). He will not [be] official U.S. Government, will not represent U.S. Government opinions, will not communicate to media or social media and will presume all information is [Sensitive but Unclassified / Not Releasable to Foreign Nationals.”
JANUARY 31, 2020. On January 31, 2020, the former Secretary, Alex M. Azar II, declared a public health emergency pursuant to section 319 of the PHS Act, 42 U.S.C. 247d, effective January 27, 2020, for the entire United States to aid in the response of the nation’s health care community to the COVID–19 outbreak
LATE JANUARY/ EARLY FEBRUARY 2020. During late January/early February 2020, Dr. Bright also launched a comprehensive review of existing drugs that had been developed for MERS, SARS, Ebola and other viruses to urgently determine if there might already be a drug available or in late stage development that could work against the novel coronavirus. …. In conducting this assessment, Dr. Bright became concerned about the limited supply of Remdesivir, a broad-spectrum antiviral medication developed by Gilead Sciences (“Gilead”) that appeared, based on limited data coming from China and some laboratory-based testing, to lower the number of days it took patients to recover from COVID-19.
FEBRUARY 01, 2020. "On February 1, 2020, Dr. Fauci, Dr. Collins, and at least eleven other scientists convened a conference call to discuss COVID-19. It was on this conference call that Drs. Fauci and Collins were first warned that COVID-19 may have leaked from the WIV and, further, may have been intentionally genetically manipulated.”
FEBRUARY 04, 2020. “Gilead Sciences has partnered with Chinese health authorities to conduct a randomised Phase III clinical trial to assess the use of antiviral drug candidate remdesivir (GS-5734) for the potential treatment of coronavirus. … In Beijing, China, the new placebo-controlled Phase III trial of Gilead’s drug will be performed at Friendship Hospital. The study is set to enroll 270 patients with mild and moderate pneumonia caused by the coronavirus.” Clinical Trials Arena. The Phase III status of the trial means that Phases I and II had begun earlier, but I do not have information on that.
FEBRUARY 06, 2020. Chinese researchers begin a study in China using Remdesivir. The study had 237 patients and ran between Feb 6, 2020, and March 12, 2020. The researchers achieved 14% death rates in the Remdesivir group, and 13% in the placebo group. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial
FEBRUARY 07, 2020. Anthony Fauci sends an email to John W. Mellors (University of Pittsburgh) following up on a shipment of Remdesivir to China, which was “on it’s way.”
FEBRUARY 10, 2020. Robert and Jill Malone publish “Novel Coronavirus: A Practical Guide for Preparation and Protection.”
FEBRUARY 11, 2020. The WHO names COVID-19.
FEBRUARY 12, 2020. Fierce Pharma reports that China has copied Remdesivir and had already mass produced it. “The Chinese pharma BrightGene has successfully copied remdesivir, the company said in a disclosure (PDF, Chinese) to China’s Nasdaq-style Star market on Wednesday. What's more, the Suzhou-based firm said it has already mass-produced remdesivir's active ingredient and is in the process of turning it into finished doses. The company’s stock jumped 20% at the news, hitting the daily price move cap allowed on the exchange.”
FEBRUARY 13, 2020. Clifford Lane, Deputy Director at NIAID, travels to China as part of a World Health Organization inspection team. Lane had been originally scheduled to travel to Japan to set up trials of Remdesivir. The Science article by Jon Cohen did not state if Lane observed Remdesivir treatments.
FEBRUARY 13, 2020. China reports 242 COVID deaths. Applied Mathematician Eva K. Lee is confused. NY TIMES RED DAWN RISING EMAILS
FEBRUARY 25, 2020. NIH clinical trial of Remdesivir to treat COVID-19 begins in Nebraska. NIH. The NIH noted that clinical trials of Remdesivir were also underway in China. The NIH noted that their Remdesivir trials were based on the Remdesivir trials in China.
FEBRUARY 26-27, 2020. The Boston Biogen Superspreader Conference. “The biotech conference would quickly become a superspreader event, launching the COVID-19 outbreak in eastern Massachusetts and causing as many as 330,000 cases of coronavirus worldwide, according to a study published in the journal Science.” NBC Boston. I have seen speculation that the virus/bioweapon was intentionally spread at this conference by coating the filters on the air systems, or by an aerosol delivery system. Given the amount of deception associated with this Pandemic, I think it is likely that a virus/bioweapon was intentionally spread at this event, although it is pure speculation.
FEBRUARY 27-28, 2020. AI Powered Drug Discovery and Manufacturing CONFERENCE 2020 MIT, Cambridge, M. MIT
LATE FEBRUARY 2020. Around this time, Robert Malone claims to have contracted COVID, and cured his COVID with Famotidine. Malone announced his results on LinkedIn in late February sometime. LinkedIn subsequently deleted his account and Malone’s announcement on his discovery of the cure for COVID. The New York Times later referenced Malone’s announcement on LinkedIn, then they also deleted their story on his discovery of Famotidine as a cure for COVID.
Malone to Joe Rogan: “Once before I was infected at the end of February, because I was attending a MIT conference on drug discovery and artificial intelligence.” Joe Rogan: “So February of 2020, you get infected. And how bad is your case?” Malone: “Bad. I thought I was going to die. You’ve got to remember, I was up up up on all the latest information from China and everywhere else. I knew all about this virus. You know I knew, I’d been watching videos of people dropping in the street. My lungs were burning until I took famotodine and that relieved that.” Joe Rogan: “And what is famotidine?” Malone: “It’s otherwise known as Pepcid. So just to, on this tangent since I’ve said it, I’ve got some good news to announce. First time here. Today we believe we should have the first patient enrolled in our clinical trials of the combination of famotidine and sylacoxic? For treating Sars-CoV-2.” (I note that the virus was never strong enough for people to drop in the street. I remember some talk on the internet that the videos of people falling down in China were faked, but if not, whatever it was wasn’t this virus. This is the type of information that our DNI should not keep from the public and Congress).
Robert Malone published More Than Just Heartburn: Does Famotidine Effectively Treat Patients with COVID-19? in February 2021. Malone said that the original plans were scrapped when insisted on using Hydroxychloroquine in the treatment arms, which would mean using HCQ with Famotidine as a treatment. “Original plans to perform an intravenous dose-ranging study in newly hospitalized patients were scrapped when the institutional review board insisted on including hydroxychloroquine in the treatment arms.” He did not explain further. He noted that the SOC had been changed, but did not note that the SOC was changed to Remdesivir. “After enrollment was initiated, changes in standard of care, Good Clinical Practice (GCP) audit irregularities, and failure to enroll adequate numbers of patients compromised that study (now listed as completed); as a likely consequence, results have yet to be reported.”
LATE FEBRUARY 2020. Robert Malone sends Chinese treatment protocols to his buddies at the CIA and DTRA. Malone: “I had Chinese connections- the Chinese protocol for treating this virus. I got it in late February and I sent it in to my buddies at the CIA and at DTRA.” - Malone on Joe Rogan.
MARCH 05, 2020. 3 people who attended the Boston Biogen Superspreader Conference test positive for COVID after developing symptoms. Mass Live
MARCH 20, 2020. On March 20, 2020, Dr. Kadlec wrote to the Executive Vice President of Research at Northwell Health, Dr. Kevin Tracey, to request an expedited review of the company’s clinical trial to develop a COVID-19 treatment. See letter from R. Kadlec to K. Tracey (Mar. 20, 2020), attached hereto as Exhibit 47. Northwell Health was working with Alchem Laboratories (“Alchem”) on a treatment using hydroxychloroquine in combination with famotidine, the active compound in the heartburn drug Pepcid AC. Dr. Kadlec invited Northwell Health to submit a proposal to ASPR Next and, in an unprecedented move, instructed it to “work with COVID clinical expert, Dr. Michael Callahan, in the preparation of this white paper and draft budget.” Id. Dr. Callahan is a consultant on Dr. Kadlec’s staff who was hired to advise HHS about the government’s COVID response. He is not a government employee. Yet as a consultant “who is advising or has advised the Federal Government with respect[] to a Federal agency procurement,” Dr. Callahan is prohibited from disclosing information about a contractor bid or proposal, or source selection information, before the award of a Federal agency procurement contract. See 41 U.S.C. § 2102(a)(3)(A). - Rick Bright Whistleblower Complaint
APRIL 01, 2020. Robert Malone writes: “…my colleague Dr. Michael Callahan ( who currently reports directly to the ASPR in US) has supervised treatment of well over 6000 cases of COVID19, was in country in the PRC assisting, …”
APRIL 07, 2020. Start date for ClinicalTrials.gov Identifier: NCT043702626 using Famotidine and Hydroxychloroquine.
APRIL 07, 2020. “On 7 April, the first COVID-19 patients at Northwell Health in the New York City area began to receive famotidine intravenously, at nine times the heartburn dose. Unlike other drugs the 23-hospital system is testing, including Regeneron's sarilumab and Gilead Sciences's remdesivir, Northwell kept the famotidine study under wraps to secure a research stockpile before other hospitals, or even the federal government, started to buy it.” Science. Brenden Borrell
“APRIL 08, 2020. Kevin Tracey (Northwell) tells CNN that results of a small study of hydroxychloroquine in France was “a complete failure.” “‘The study was a complete failure,’ he said. ‘It was pathetic,’ added Art Caplan, head of the division of medical ethics at the New York University School of Medicine. The small French study of 20 people found that taking hydroxychloroquine was associated with the ‘viral load reduction/disappearance in COVID-19 patients,’ noting that the effect was ‘reinforced’ with azithromycin, an antibiotic better known as a Z-pack.’
Below is a screenshot of the results of the French hydroxychloroquine study that Kevin Tracey of Northwell mentioned to CNN. It seemed to report good results, far better than anything with Remdesivir. I did not see any reports of death in the French study. Tracey’s comments reflect that he deliberately mischaracterized the results of hydroxychloroquine and azithromycin. Soon after, Tracey would receive a grant to test hydroxychloroquine and famotidine. Hydroxychloroquine was replaced with the deadly Remdesivir.
APRIL 10, 2020. Gilead Sciences makes a press release on their 13% death rate in their China Remdesivir trial, which they call a “clinical improvement.” They said the death rates were greater in the group on ventilators. It seems very likely to me that these “clinical researchers” can achieve whatever mortality rates they want with the use of ventilators.
APRIL 13, 2020. Gilead suspends their Remdesivir trials in China, Study # NCT04252664. Gilead said “The epidemic of COVID-19 has been controlled well at present, no eligible patients can be recruitted.)" The study was likely suspended due to the high death rate. They later said they couldn’t get enough patients, and made no mention of their comment that “The epidemic of COVID-19 has been controlled.”
APRIL 21, 2020. Around this time, Robert Malone resigned from Alchem and expressed dissatisfaction with the Hydroxychloroquine/Famotidine trial and Michael Callahan, according to AP News:
APRIL 23, 2020. Gilead Sciences posts an update on their suspension of their Remdesivir clinical trial in China. Gilead said they stopped the study due to low enrollment, and that the WHO did not have their permission to report the data. Gilead: “Today, information from the first clinical study evaluating the investigational antiviral remdesivir in patients with severe COVID-19 disease in China was prematurely posted on the World Health Organization website. This information has since been removed, as the study investigators did not provide permission for the publication of the results. Furthermore, we believe the post included inappropriate characterizations of the study. The study was terminated early due to low enrollment and, as a result, it was underpowered to enable statistically meaningful conclusions.” So it looks like there were a few good people at the WHO, but Gilead had enough power to have the WHO’s assessment of Remdesivir removed.
APRIL 27, 2020. Business Insider Article publishes interview with Kevin Tracey. Tracey said that the information on Famotidine came from Michael Callahan in Wuhan. Again, Callahan was likely administering Famotidine in Wuhan. How he decided to use Famotidine in Wuhan is unclear. The DOMANE program seemed to be online back in December 2019, but researchers in the United States were not reported to have the virus sequence before January 10, 2020.
Tracey said that Northwell’s 23 hospitals were at the center of the outbreak. The “time” referred to is unclear. It is not clear if the “Boston superspreader conference” was the origin of the outbreak managed by Northwell. Tracey said there was “no clinically proven therapy.” If hydroxychloroquine had been proven in clinical trials to be effective, as an existing FDA-approved therapeutic, the Emergency Use Authorizations for Remdesivir and the mRNA vaccines could not have happened.
At this point Kevin Tracey was aware that the trial he would be running used Hydroxychloroquine.
Tracey said he didn’t want to use Hydroxychloroquine. This is evidence that he did not want to provide evidence that would be used to approve Hydroxychloroquine for COVID.
Kevin Tracey said he wanted to add another treatment arm of Famotidine without Hydroxychloroquine. They did not test Famotidine alone, however. After the Standard of Care was changed on April 29 to Remdesivir, they tested Remdesivir + Famotidine vs. Remdesivir + Placebo.
Dr. Thomas Maddox made a comment that I think describes the key to the evil methods that have been used to approve toxins and block approval of effective medicines for decades. “Control” is the key. You have to kill more in Group B than Group A to get the results you want. And that’s exactly what Kevin Tracey et al did at Northwell.
APRIL 28, 2020. Robert Malone tweets a “press release” about the clinical trials award of Hydroxychloroquine and Famotidine to Alchem. (The link to Malone’s website does not function).
According to Robert Malone’s CV, he was a consultant for Alchem and/or its CEO from 2012 to 2019, then became Chief Medical Officer from November 2019 through April 2020. (He resigned in April). At Alchem, Robert Malone led “through-put screening” that identified Famotidine. Malone was the technical Lead/writer for the Famotidine/Hydroxychloroquine clinical trial.
APRIL 29, 2020. Gilead Sciences announces results of clinical trials using Remdesivir. They said that they killed 23 out of 320 patients, or 7.2%. This would become the new Standard of Care (SOC) in the Hydroxychloroquine/Famotidine trials, replacing Hydroxychloroquine.
Gilead said “No new safety signals were identified.” Some old safety signals were identified however, apparently. In addition to “death,” which Gilead does not list as a side effect, Gilead also mentioned nausea, acute respiratory failure, and grade 3 or higher liver enzyme (ALT) elevations associated with Remdesivir.
APRIL 29, 2020. Anthony Fauci said that the 7% death rate results of Gilead’s Remdesivir study were “quite good news” and that Remdesivir would become the new standard of care (SOC). A report from CNBC enumerated a higher death rate than Gilead did in their press release. While Gilead reported 7% death rate in both treatment groups, CNBC reported 11.6% death in the “placebo” group and 8% in the “Remdesivir group.” That was a “benefit,” not killing people in both groups. I hate to think what they did to the placebo group.
Barry Zingman, who led Remdesivir trials at Montefiore-Einstein in New York, spoke of why the results of NCT04280705 https://clinicaltrials.gov/ct2/show/NCT04280705 were announced early and they "broke the blind." They said the reason was because the results were so good, that it would be unethical to continue with placebo. I think the reality is that they were concerned about the hydroxycholorquine/famotidine trials, and wanted an excuse to stop using hydroxychloroquine. They said there was a mortality benefit, which only means that they somehow killed more people in the placebo group. Here are some comments from Barry Zingman, who led the trial:
"The reason why the results were announced last week was that an independent group of statisticians and clinicians who were evaluating the safety and effectiveness of the treatment determined that there was such significant differences between the treatment arm and the placebo arm was that it would be unethical to continue to give only placebo after the results were presented. After the results were presented to the FDA we were able to what's called "break the blind." To look at all of the patients who were still hospitalized so I could then ask the research Pharmacist if they had previously gotten placebo or Remdesivir. If they had gotten placebo, I could then offer them Remdesivir."
MAY 01, 2020. Around this time, the standard of care (SOC) on ClinicalTrials.gov NCT04370262 Alchem/Northwell Northwell Famotidine/Hydroxychloroquine trials was changed from Hydroxychloroquine to Remdesivir.
MAY 23, 2020. Robert Malone writes a preprint of intentions to do a Famotidine trial. He mentions the results of the Northwell trials, but does not mention that Remdesivir was used in the trials. “A retrospective cohort study of 1,620 hospitalized COVID-19 patients indicates that 84 propensity score matched patients receiving famotidine during hospitalization (oral or IV, 20mg or 40mg daily) had a statistically significant reduced risk for death or intubation..” As Remdesivir was the primary cause of death in the Northwell trials, the results of the Northwell trial showing a reduction in “COVID” deaths due to Famotidine could not be considered to be statistically valid.
JULY 24, 2020. Robert Malone et al submit results for publication of their Famotidine/Celecoxib trial. Hospitalized COVID-19 Patients Treated With Celecoxib and High Dose Famotidine Adjuvant Therapy Show Significant Clinical Responses
It is interesting that the trial was not Famotidine/Hydroxychloroquine. Malone et al reported a 100% survival rate, and good results overall. They did not use ventilators. They did not report a placebo or control group.
SUMMARY.
I believe that there is more than enough evidence for the New York State Attorney General to begin a criminal investigation into Kevin Tracey and Michael Callahan’s role in intentionally killing 238 COVID patients in the Northwell “Famotidine” clinical trials with Remdesivir protocols. As for Dr. Malone, I don’t know if he will agree with my conclusions or not. Considering Malone’s lead role at Alchem in designing the Hydroxychloroquine/Famotidine trials, his resignation, and the subsequent change in the Standard of Care from Hydroxychloroquine to Remdesivir, I believe it is appropriate for Dr. Malone to address what I consider to be matters of fact reported here related to Kevin Tracey, Michael Callahan, and Remdesivir and Northwell’s clinical trials. His expertise is well beyond mine on this subject.
Charles Wright






































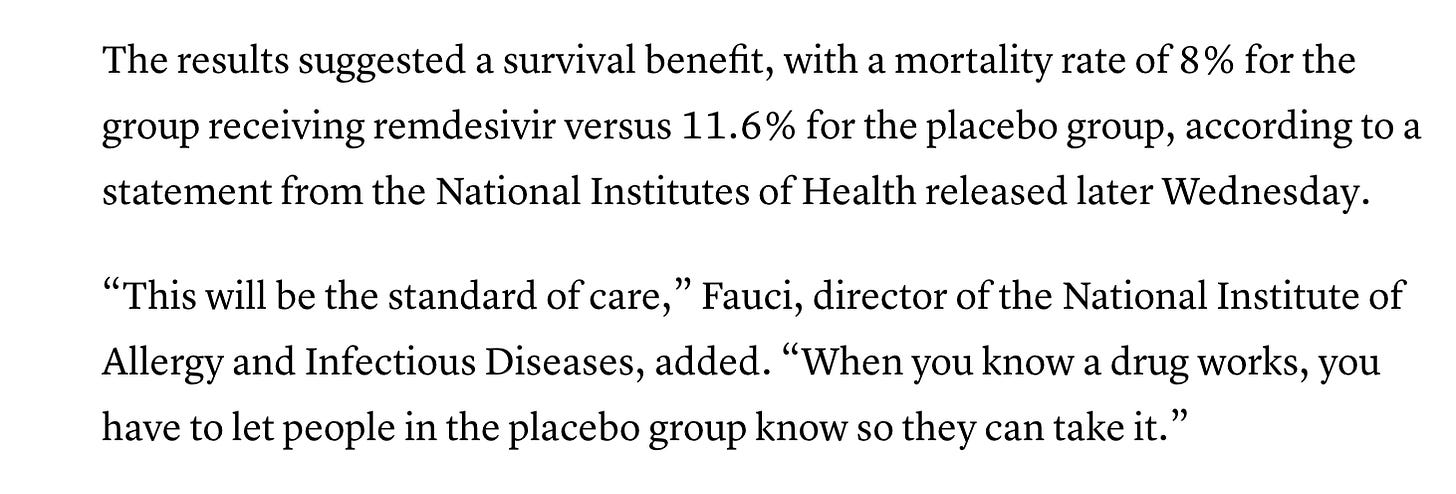




I remember discussing early on about how they put people on ventilators but they were dying. We were wondering why they kept doing it. At that point we didn 't know about remdesivir.
Breathtaking!