This article will highlight the importance of checking into the actual causes of deaths said to be associated with alleged viruses. For instance, a “WEF Doctor,” Leana Wen, has recently warned that H5N1 will kill 52% of humans unless the public takes vaccines. Wen: “I feel like we should have learned our lesson from COVID. That just because we aren’t testing, it doesn’t mean that the virus isn’t there.”
Read more about Wen in Capt. Roy Harness’ Substack.
I did learn my lesson from COVID. Dr. Wen. People died in hospitals after countermeasures were implemented based on PCR testing. The state of New York designed and implemented their own PCR test after the CDC PCR test returned 100% negative results to them. New York rapidly scaled up PCR testing to over 10,000 per day and claimed a high percentage of positive tests. Patients were subsequently killed in very large numbers in hospitals with protocols that were clearly designed to kill.
And so I wondered, just how accurate is it that an alleged virus ever caused the deaths attributed to H5N1?
In this article, I will partially document the chronology of the discovery of the alleged H5N1 virus and the deaths that were said to be caused by it between 1997 and 2006. Then I will look at the actual H5N1 cases in hospitalized patients.
According to an archived webpage of the CDC, H5N1 was first “identified” in a goose in Guandong, China, in 1996 (1). The "virus” was first named “A Goose Guandong 1 1996.”
The CDC said H5N1 jumped to people in Hong Kong in 1997, causing 18 cases and 6 deaths. (1). Intermediately, there was a chicken.
Some background: The People’s Republic of China was formed in 1949 under Mao Zedong, Chairman of the Communist Party in China. Zedong implemented Kill Quotas of 1 per 1000 people in rural areas and 1 per 2000 in urban areas in 1951. China implemented a mass starvation program between 1959-1961 known as the “Great Famine,” which starved tens of millions of people to death. China implemented a “One Child” per family program from 1979-2015.
Hong Kong became a possession of Britain in 1841.
In 1997 Hong Kong was one of the most densely-populated regions of the world with over 6 million people living on the island.
Hong Kong has a thriving “wet market” industry. A wet market is where fresh produce, poultry, pork and foods are sold, as opposed to a dry market where frozen food is sold.
Just prior to return of rule of Hong Kong to China, the first alleged case of H5N1 was in May 9, 1997 in an healthy 3 year-old boy in Hong Kong who had developed fever, sore throat, and cough. (3). The child “may have been exposed to chickens,” according to the CDC. (3).
Oddly enough, rule of Hong Kong was formally returned from Great Britain to China on July 1, 1997, when Hong Kong became a “special administrative region” of China. A ceremony was held on June 30.
The Chinese boy soon died from “respiratory failure,” according to the CDC. (5). Claas et al, in the Lancent in February 1998, elaborated further on the young boy’s cause of death. They said the boy died from Acute Respiratory Distress Syndrome (ARDS), multiorgan failure, Reye's syndrome, and disseminated intravascular coagulation. (7). Claas et al also said that the alleged “virus”that killed the 3 year-old was closer to “a chicken” virus rather than “a goose” virus according to their analysis. (10).
The death of the 3 year-old boy in Hong Kong triggered a huge epidemiological investigation.
According to the CDC, Chinese scientists claimed to have “isolated” an alleged Influenza A virus that they claimed was responsiblle for the boy’s death. (4). The Chinese scientists could not further classify the alleged Influenza A virus, so they sent samples to the US CDC, the Netherlands, and the United Kingdom. Scientists subsequently grew an alleged virus in Madrin-Darby Canine Kidney (MDCK) epithelial cells, and classified it as Influenza A- H5N1. (4).
In August 1997, 502 serum samples were taken in Hong Kong from family members of the boy, poultry farmers, pig farmers, medical workers, and others in proximal location to the boy in order to test for “H5N1” antibodies. 9 of the 502 samples were positive. (8). Based on the gathered serologic data, the CDC said “the virus probably is not being efficiently transmitted among humans.” (9).
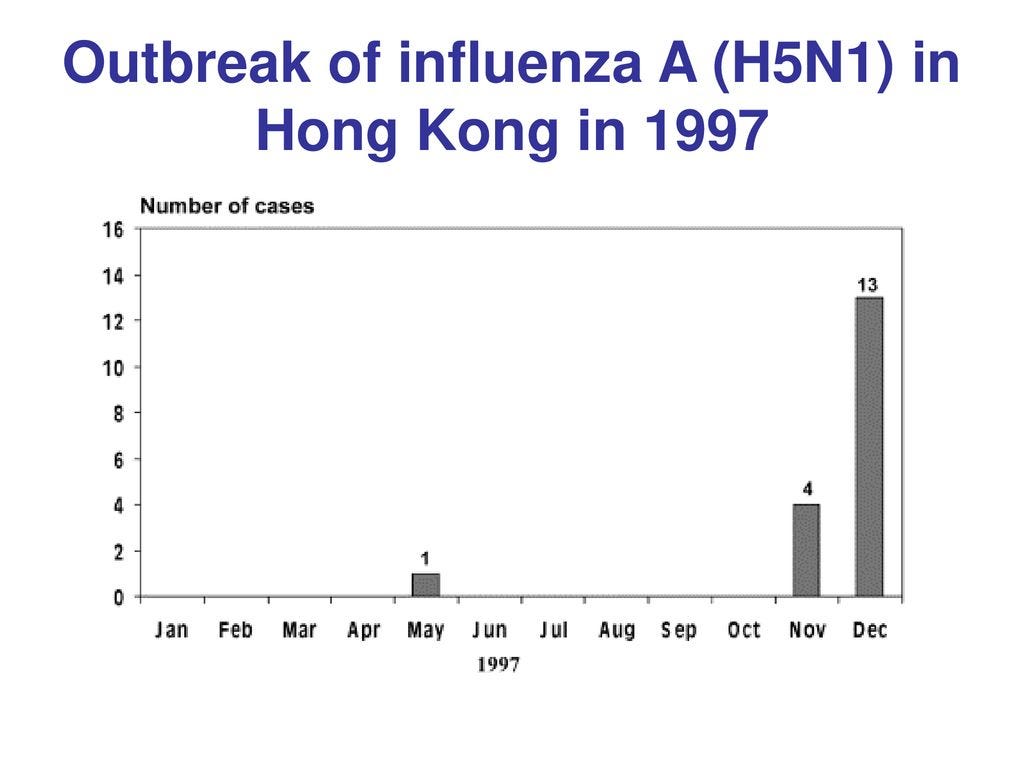
There were no more “H5N1” cases in Hong Kong until November 1997.
In November 1997, four people developed sore throat, coughing, and fever in Hong Kong. They were also said to have pneumonia. (6)
13 were said to have acquired H5N1 in Hong Kong in December 1997, but these cases are less documented by the CDC than the cases in November based on the material I have reviewed. (6).
According to a paper by Paul Chan, the alleged H5N1 virus cases and deaths were caused by chickens, and the alleged outbreak was stopped by killing 1.5 million chickens at the end of December 1997. Chan said the clinical symptoms of H5N1 ranged from asymptomatic to multiple organ failure and fatal pneumonia, and that PCR tests and immunoflourescent assays were used to rapidly identify the alleged virus. (2).
Shortridge in 1997 said the mass slaughter of all chickens in Hong Kong began on December 29, 1997. (11). The last alleged case of H5N1 in Hong Kong was on December 28, 1997. Shortridge said the lack of H5N1 cases after the slaughter of all the chickens in Hong Kong in December 1997 “vindicated” the action.
After 1997, Hong Kong’s new Chinese government set up a “Farm Hygiene section” to conduct testing for H5N1. (12).
Afterwards, the alleged Goose/Chicken H5N1 virus lay dormant. In 2001-2003, H5N1 was said to reemerge, but only in chickens (except for 2 cases in humans). Of course, China killed the chickens again.
The CDC reported that H5N1 was detected in retail markets in China in 2001, but was not detected initially in poultry farms. (13). China first slaughtered all the live chickens in the live markets in 2001.
In February 2002, Hong Kong traced back a case of “H5N1” from a chicken in a live market back to the farm it came from. By late March 2002, China had identified 17 chicken farms that they said were infected. China combatted the alleged H5N1 virus with “depopulation of infected and contact farms, quarantine and enhanced biosecurity, and vaccination.” (13).
Despite the “poultry outbreaks" in Hong Kong that led to the destruction of chickens, there were only two cases of “H5N1” reported in Hong Kong in from 2003-2006, both in 2003. One was fatal. (14).
There were other cases of “H5N1” and deaths reported in multiple other countries, however. (16). The image below of H5N1 cases and deaths is from the World Health Organization.
The most reported cases and deaths were in Vietnam, followed by Indonesia, Thailand, Turkey, China, Cambodia, and Iraq.
The two cases with one fatality from Hong Kong were not reported in this chart above from the WHO, although the source on the two cases of H5N1 with one fatality in Hong Kong was also the WHO, for some odd reason.
Of course, by 2003 there was a new “virus” going around in Asia, SARS-CoV-1, which was said to have infected 8,098 people, killing 774 in 2003. (15). SARS1 was also said to hit Hong Kong. In other words the names of alleged viruses that were responsible for deaths were simply changed.
In December 2005, Crowe and Engelbrecht published: Avian flu virus H5N1: No proof for existence, pathogenicity, or pandemic potential; non-“H5N1” causation omitted.
The rest of this article will review what the actual cause of the “H5N1” deaths actually were between 1997 and 2006.
The rest of the article is a paid section. I put about 40 hours of research and writing into this, so I’d like to get something out of it.
The deaths attributed to an alleged H5N1 virus 1997-2006 were isolated, sporadic deaths due to medical malpractice. This is why in the literature, authors claim that the “virus” did not spread well between humans, or that it spread asymptomatically.
The H5N1 patients all presented intially with influenza or pneumonia, then they were killed in the hospitals. H5N1 virus was simply a scapegoat for the deaths via medical malpractice. Whether or not the H5N1 virus was ever adequately isolated and sequenced, or whether H5N1 could have caused the symptoms in the patients said to have H5N1 is another question. The methodology used to substantiate the existence of an alleged H5N1 virus is dubious at best, but the actual cause of death is clear.
The best information I have found on the actual cause of “H5N1” deaths was written by Pascale C Gruber, Charles D Gomersall, and Gavin M Joynt in March 2006. (16).
At the time of the publication of their paper in 2006, there were 165 cases and 88 deaths attributed to H5N1, according to the WHO, as referenced in the chart above.
These H5N1 cases and deaths were so spread out over time and distance that they were difficult to analyze. The authors had to review multiple papers to make their findings. The authors searched for the terms “‘avian influenza’ and ‘H5N1’ for human studies published after 1996 in English.”
They searched for and recorded the following information where possible: “age, sex, hospital mortality, length of ICU stay, duration of symptoms before hospital admission, and duration of hospital stay before requirement for advanced organ support. The presence of respiratory, cardiovascular, renal, hepatic, haematological, central nervous system, gastrointestinal and multiorgan failure, acute respiratory distress syndrome (ARDS) and pneumothorax was recorded.”
Of the 188 cases reported by the WHO 1996-2006, the authors only found recorded data on 65 of the patients. There is more data that could be gathered by searching papers written in languages other than English.
Nevertheless, the data they gathered was very informative.
44 of the 65 patients required “advanced organ support” or “advanced life support.” The authors used both of these terms in the paper. Based on my read of the paper, those terms mean the same thing and were used interchangeably.
Advanced organ support was “defined as invasive mechanical ventilation or administration of inotropes or vasopressors.”
Here is a description of inotropes and vasopressors:
Inotropes and vasopressors are essential pharmacological agents used to treat shock, a condition characterized by reduced perfusion to vital organs, leading to multiorgan dysfunction and potentially death. Vasopressors function by inducing vasoconstriction, thereby increasing systemic vascular resistance (SVR), mean arterial pressure (MAP), and organ blood flow. Inotropes enhance cardiac contractility and improve cardiac output (CO), which supports the maintenance of MAP and perfusion. These agents are vital for restoring hemodynamic stability in critically ill patients.
The authors did not break the data down between which of the 44 patients received mechanical ventilation, inotropes, ventilators, or combinations of the 3. They also did not report the mortality rate of the 21 of 65 “H5N1” patients who did not receive “advanced life support.” That data would be informative, especially given that the ones who did not receive “life support” very likely died at much lower rates than those who did receive “life support.”
The authors reported that 41 of the 44 who received “advanced life support” died. Now if that’s “life support,” why did they die at such a high rate? I don’t think “life support” is an accurate term for the methods used here. I think the term they should use is “method and cause of death.”
The authors said that ARDS was very common. ARDS was specifically identified in 54% of the 41 patients who receive advanced organ support. The authors felt like the ARDS rate in the 41 patients was much higher than 54%, but that the papers they reviewed did not provide enough detail to determine whether ARDS was present in those patients who were reported to have “multiorgan failure” without further detail.
Of the 41 patients who required advanced organ support 22 (54%) developed ARDS, but this is likely to be an underestimate. Many patients with severe respiratory failure were reported with insufficient detail to establish whether they had ARDS. …
These figures only provide a rough estimate as in many cases definitions of organ failures or ARDS were not documented, and patients were reported as having multiorgan failure without specific organ failures being given.
The authors said that pneumothorax was present in many patients, and that pneumothorax “occurred during mechanical ventilation.”
Pneumothorax was common (17%). All pneumothoraces occurred during mechanical ventilation.
What is a pneumothorax?
A pneumothorax is an abnormal collection of air in the pleural space between the lung and the chest wall.[3] Symptoms typically include sudden onset of sharp, one-sided chest pain and shortness of breath.[2] In a minority of cases, a one-way valve is formed by an area of damaged tissue, and the amount of air in the space between chest wall and lungs increases; this is called a tension pneumothorax.[3] This can cause a steadily worsening oxygen shortage and low blood pressure. This leads to a type of shock called obstructive shock, which can be fatal unless reversed.[3] Very rarely, both lungs may be affected by a pneumothorax.[6]
Thus, the use mechanical ventilators would have caused pneumothorax and “obstructive shock.”
As I referenced earlier, “Inotropes and vasopressors are essential pharmacological agents used to treat shock, a condition characterized by reduced perfusion to vital organs, leading to multiorgan dysfunction and potentially death.”
So that’s apparently why the medical professionals were using inotropes and vasopressors. Inotropes and vasopressors were not used to treat influenza or pneumonia; they were used to treat pneumothorax and obstructive shock caused by mechanical ventilators.
Therefore, the data suggests to me that all 44 patients received mechanical ventilation, and that inotropes and vasopressors were used to treat the complications caused by the mechanical ventilators in some of the patients. Further research is required on this detail.
Also note that ARDS was not a reported symptom in the “H5N1” patients in Hong Kong in 1997. Rather, these patients were said to have fever, sore throat, coughing, and/or pneumonia. Therefore, the data suggests strongly that ARDS was also caused by mechanical ventilation.
To support this hypothesis, note that the authors specifically said that the “H5N1” patients “developed” ARDS- not that they initially presented with ARDS.
Of the 41 patients who required advanced organ support 22 (54%) developed ARDS, but this is likely to be an underestimate.
Even the authors were concerned about the use of ventilators and suggested using a different ventilation strategy.
The high incidence of ARDS and pneumothorax has implications for the type of ventilators that should be stockpiled for use in an epidemic and ventilatory management. A low-volume low-pressure strategy for ventilation of patients with ARDS has been shown to reduce mortality with a number needed to treat of 4.52 [37].
Essentially, the authors said in a far-too polite manner that medical professionals killed their patients with ventilators. They said that a different ventilation strategy would “reduce mortality,” which I’m sure it would, considering that there’s not much room to do worse than killing 41 out of 44 (93%).
The authors got this part very, very wrong in my opinion:
Severe avian influenza causes a rapidly progressive disease that often culminates in ARDS, multiorgan failure and death.
An alleged avian influenza virus did not cause ARDS, multiorgan failure, and death in H5N1 patients between 1996-2006. Medical professsionals did that- primarily with their use of mechanical ventilators.
CONCLUSION
Every “viral pandemic” that I have reviewed thus far that was said to have caused a high death rate, or a large amounts of death, shows that the deaths were among those who were ventilated. Those pandemics include Nipah virus, (18, 19), H5N1, SARS1 (20, 21), and SARS2 (22, 23, 24, 25, 26) at a minimum. Even the “iron lungs,” the first mechanical ventilators which were used to treat poliomyelitis, had an initial mortality rate of 90% which fell to 20% after the design of the iron lung was changed. (17).
In my opinion, H5N1 began as an indirect stratety to control population growth in Hong Kong by destroying a vital part of their food supply. Destroying the food supply via alleged and unsubstantiated viruses is still used today with H5N1 all over the world, including in the United States.
The strategy to kill humans directly with alleged viruses in hospitals became transparent when SARS (Sudden Acute Respiratory Syndrome) was name after ARDS (Acute Respiratory Distress Syndrome) caused by ventilators. Quite simply, a virus wa named after the methods used by hospitals to kill patients. After naming a virus after ARDS, people would be far less likely to question why the patients were all dying from ARDS instead of fever, cough, and normal symptoms of influenza. The virus did it. It’s in the name of the virus. Unfortunately it was a strategy that worked on a huge scale all over the world from 2019-present.
This direct depopulation strategy via alleged viruses, that were actually hospital murder, was greatly expanded in SARS2. In SARS2 tremendous amounts of PCR tests were used that led to death by hospital protocols and vaccines. Great amounts of deadly drugs such as Remdesivir, Midazolam, tranquilizers, and opiates were also included in the deadly SARS2 hospital protocols. In addition to the ARDS caused by mechanical ventilators, many also died of bacterial sepsis and blood clots caused by the ventilators. These methods of death were also blamed on an alleged virus never isolated to be sequenced to begin with.
It’s time for medical professionals to report the actual causes of deaths using their own research instead of repeating that the causes of death can only be attributed to the results of a PCR test du jour, as they read in most medical literature without bothering to question it.
REFERENCES
(1). Emergence and Evolution of H5N1 Bird Flu, CDC.gov, 2022. https://web.archive.org/web/20220605042034/https://www.cdc.gov/flu/pdf/avianflu/bird-flu-origin-graphic.pdf
In 1996, highly pathogenic avian influenza H5N1 virus is first identified in domestic waterfowl in Southern China. The virus is named A/goose/Guangdong/1/1996. In 1997, H5N1 poultry outbreaks happen in China and Hong Kong with 18 associated human cases (6 deaths) in Hong Kong.
(2). Outbreak of Avian Influenza A (H5N1) Virus Infection in Hong Kong in 1997. https://www.jstor.org/stable/4483086
The first outbreak of avian influenza A (H5N1) virus occurred in Hong Kong in 1997. Infection was confirmed in 18 individuals, 6 of whom died. Infections were acquired by humans directly from chickens, without the involvement of an intermediate host. The outbreak was halted by a territory-wide slaughter of more than 1.5 million chickens at the end of December 1997. The clinical spectrum of H5N1 infection ranges from asympomatic infection to fatal pneumonia and multiple organ failure. …
Rapid diagnosis with the use of reverse-transcription polymerase chain reactions and monoclonal antibody-based immunoflourescent assay were of great clinical value in the management of the outbreak.
(3). Isolation of Avian Influenza A(H5N1) Viruses from Humans -- Hong Kong, May-December 1997, CDC MMWR, December 19, 1997. https://www.cdc.gov/mmwr/preview/mmwrhtml/00050459.htm
Patient 1. On May 9, 1997, a previously healthy 3-year-old boy developed fever, sore throat, and cough. The child's symptoms persisted, and on May 15, he was hospitalized. His illness progressed, and on May 18, he was admitted to the pediatric intensive care unit (ICU). On May 21, the child died from acute respiratory distress secondary to viral pneumonia. Influenza A(H5N1) virus was isolated from a tracheal aspirate collected on May 19. The child may have been exposed to ill chickens before he became ill.
(4). OUTBREAK IN HONG KONG, 1997, CDC Museum webpage. https://www.cdcmuseum.org/exhibits/show/influenza/avian-influenza/outbreak-hong-kong
(5). Update: Isolation of Avian Influenza A(H5N1) Viruses from Humans -- Hong Kong, 1997-1998. CDC MMWR, January 9, 1998. https://www.cdc.gov/mmwr/preview/mmwrhtml/00050775.htm
The first known case of human infection with influenza A(H5N1) occurred in a 3-year-old boy who died from respiratory failure in May 1997 (1).
(6). Isolation of Avian Influenza A(H5N1) Viruses from Humans -- Hong Kong, May-December 1997, CDC MMWR, December 19, 1997. https://www.cdc.gov/mmwr/preview/mmwrhtml/00050459.htm
Patient 2. On November 6, a 2-year-old boy with a congenital heart disease developed high fever, cough, and sore throat and was hospitalized the next day for presumed pneumonia. He had an uneventful recovery and was discharged from the hospital on November 9. A nasopharyngeal swab collected from the child on November 8 yielded influenza A(H5N1) virus.
Patient 3. On November 20, a previously healthy 13-year-old girl developed fever, sore throat, and cough; she was hospitalized on November 26 because of pneumonia. On November 27, she was transferred to the ICU and placed on mechanical ventilation. As of December 17, she remained hospitalized. Influenza A(H5N1) virus was isolated from a tracheal aspirate collected on November 28.
Patient 4. On November 24, a previously healthy 54-year-old man developed fever and cough and on November 29, he was hospitalized because of pneumonia. His condition deteriorated, and he died on December 5. A broncho-alveolar lavage specimen collected on December 1 yielded influenza A(H5N1) virus. …
On November 24, a previously healthy 37-year-old man was hospitalized because of pneumonia; onset of illness was November 17. He recovered and was discharged from the hospital on December 9. Although respiratory specimens were unavailable for testing, preliminary results of serologic tests suggest infection with influenza A(H5N1); results of a neutralization assay, which is required to confirm infection, are pending.
Patient 5. On December 4, a 24-year-old woman developed fever, sore throat, cough, and dizziness. Her symptoms worsened, and she was hospitalized on December 7. Her condition deteriorated, and on December 9, she was transferred to the ICU and placed on mechanical ventilation; as of December 17, she remained in the ICU. Influenza A(H5N1) was isolated from a tracheal aspirate collected on December 9.
Patient 6. On December 7, a 5-year-old girl developed fever, rhinitis, cough, sore throat, and vomiting. As of December 17, she remained hospitalized in satisfactory and stable condition. A nasopharyngeal aspirate collected on December 10 yielded influenza A(H5N1).
Patient 7. On December 12, a 2-year-old boy developed fever and was admitted to the hospital in good condition. The child is a cousin of patient 6, who frequently visited him and his family at their home. On December 16, a culture from the child was reported positive for influenza A(H5N1) virus. Possible Cases
(7). Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus, Claas et al, Lancet, February 14, 1998. https://pubmed.ncbi.nlm.nih.gov/9482438/#:~:text=Background:%20In%20May%2C%201997%2C,Hong%20Kong/258/97.
Background: In May, 1997, a 3-year-old boy in Hong Kong was admitted to the hospital and subsequently died from influenza pneumonia, acute respiratory distress syndrome, Reye's syndrome, multiorgan failure, and disseminated intravascular coagulation. An influenza A H5N1 virus was isolated from a tracheal aspirate of the boy. Preceding this incident, avian influenza outbreaks of high mortality were reported from three chicken farms in Hong Kong, and the virus involved was also found to be of the H5 subtype.
Methods: We carried out an antigenic and molecular comparison of the influenza A H5N1 virus isolated from the boy with one of the viruses isolated from outbreaks of avian influenza by haemagglutination-inhibition and neuraminidase-inhibition assays and nucleotide sequence analysis.
Findings: Differences were observed in the antigenic reactivities of the viruses by the haemagglutination-inhibition assay. However, nucleotide sequence analysis of all gene segments revealed that the human virus A/Hong Kong/156/97 was genetically closely related to the avian A/chicken/Hong Kong/258/97.
(8).
Testing has been completed of serum samples collected in August as a part of the epidemiologic investigation of the first case of human influenza A(H5N1) infection. Serum samples were obtained from 502 persons who may have had contact with the child or with poultry, including family members, persons who lived in the same neighborhood, children and staff of the child-care center the child attended, health-care workers, poultry workers, and persons working on pig farms. Samples of control serum specimens were obtained from 218 healthy children and 201 healthy adult residents of Hong Kong. These samples were tested for antibody to influenza A(H5N1) virus using a micro-neutralization assay. Of the 502 persons tested who may have had contact with the child or with poultry, elevated neutralization antibody titers to influenza A(H5N1) virus were present in nine (2%). These persons included five (17%) of 29 poultry workers, one (2%) of 54 health-care workers, one (2%) of 63 neighbors, one (1%) of 73 laboratory workers, and one (0.4%) of 261 child-care center contacts. Specimens were negative for the four family members, 18 persons working on pig farms, and the 419 controls. Seropositivity was not associated with reported ILI.
(9). Update: Isolation of Avian Influenza A(H5N1) Viruses from Humans -- Hong Kong, 1997-1998. CDC MMWR, January 9, 1998. https://www.cdc.gov/mmwr/preview/mmwrhtml/00050775.htm
The serologic data obtained as part of the epidemiologic study of the initial case support the preliminary conclusion that persons with high levels of exposure to infected poultry or direct exposure to the virus in the laboratory may be at increased risk for infection with influenza A(H5N1) virus. However, the investigation has not ruled out the possibility of person-to-person transmission from exposure to ill and infectious persons: two seropositive persons who had contact with the first case-patient included a child-care center classmate and a health-care worker, and the classmate had contact with both the ill child and the same potential environmental source of exposure to ill chickens at the school as the ill child. However, the health-care worker reported no history of exposure to the virus in the laboratory or any recent exposure to poultry, and a history of exposure to the child or to poultry was unknown for a seropositive elderly neighbor. On the basis of the overall low rates of infection among contacts and controls and the lack of seropositivity among family members, at this time, the virus probably is not being efficiently transmitted among humans.
(10). Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus, Claas et al, Lancet, February 14, 1998. https://pubmed.ncbi.nlm.nih.gov/9482438/#:~:text=Background:%20In%20May%2C%201997%2C,Hong%20Kong/258/97.
Differences were observed in the antigenic reactivities of the viruses by the haemagglutination-inhibition assay. However, nucleotide sequence analysis of all gene segments revealed that the human virus A/Hong Kong/156/97 was genetically closely related to the avian A/chicken/Hong Kong/258/97.
11. Poultry and the influenza H5N1 outbreak in Hong Kong, 1997: Abridged chronology and virus isolation, Shortridge, July 30, 1999. https://www.sciencedirect.com/science/article/abs/pii/S0264410X99001024?via%3Dihub
At the time of writing, there have been no further cases of H5N1 human influenza since the mass slaughter which commenced on 29 December 1997. This situation is seen as vindication of that action.
12. Risk for Infection with Highly Pathogenic Influenza A Virus (H5N1) in Chickens, Hong Kong, 2002, CDC, March 2007. https://wwwnc.cdc.gov/eid/article/13/3/06-0365_article#r4
After 1997, the Hong Kong government set up a Farm Hygiene section under the Agriculture, Fishery, and Conservation Department for local poultry farm surveillance. This entailed monthly testing for avian influenza and Newcastle disease viruses, testing for serologic evidence of influenza A (H5), and on-farm monitoring of disease and production. Discovery of influenza A (H5N1) in retail poultry markets triggered trace-back, which identified clinically affected farms and led to intensive on-farm investigations that identified more infected farms.
During the 2002 outbreak, clinical disease and influenza A (H5N1) isolations occurred on 22 of the 146 active chicken farms in Hong Kong. For our study, case farms were defined as farms that had high death rates caused by influenza A (H5N1) infection or farms where influenza A (H5N1) was isolated from chickens during the outbreak. Each unaffected farm (n = 124) was assigned a unique identification number, and 46 were selected by using numbers generated with a random number generator in Microsoft (Redmond, WA, USA) Excel for Windows.
13. Risk for Infection with Highly Pathogenic Influenza A Virus (H5N1) in Chickens, Hong Kong, 2002, CDC, March 2007. https://wwwnc.cdc.gov/eid/article/13/3/06-0365_article#r4
No further outbreaks of influenza A (H5N1) in poultry were recorded until 2001, when the virus was detected in live poultry retail markets in Hong Kong. Poultry farms were unaffected. This outbreak led to a slaughter of poultry in live poultry markets in Hong Kong. However, in January 2002, influenza A (H5N1) was again detected in Hong Kong wholesale and retail poultry markets (3,9). Trace-back from the wholesale poultry market led to detection of the virus on February 1, 2002, on a chicken farm in a densely populated chicken farming area of the New Territories area of Hong Kong. By late March, 17 chicken farms located within 2 km of the index farm, and 4 farms located within 2 to 5 km, were confirmed as infected (5,6). This outbreak was controlled by a combination of depopulation of infected and contact farms, quarantine and enhanced biosecurity, and vaccination (10).
14. H5N1 avian influenza: timeline, World Health Organization, October 28, 2005. https://web.archive.org/web/20110727011806/http://www.who.int/csr/disease/avian_influenza/Timeline_28_10a.pdf
Two cases of H5N1 (one fatal) are confirmed in a Hong Kong family with a recent travel history to Fujian Province, China. A third family member died of severe respiratory disease while in mainland China, but no samples were taken.
15. SARS Basics Fact Sheet, CDC. https://archive.cdc.gov/www_cdc_gov/sars/about/fs-sars.html
Severe acute respiratory syndrome (SARS) is a viral respiratory illness caused by a coronavirus, called SARS-associated coronavirus (SARS-CoV). SARS was first reported in Asia in February 2003. Over the next few months, the illness spread to more than two dozen countries in North America, South America, Europe, and Asia before the SARS global outbreak of 2003 was contained. This fact sheet gives basic information about the illness and what CDC did to control SARS in the United States. The SARS outbreak of 2003
According to the World Health Organization (WHO), a total of 8,098 people worldwide became sick with SARS during the 2003 outbreak. Of these, 774 died. In the United States, only eight people had laboratory evidence of SARS-CoV infection. All of these people had traveled to other parts of the world where SARS was spreading. SARS did not spread more widely in the community in the United States.
16. March 2006. Avian influenza (H5N1): implications for intensive care. Pascale C Gruber 1, Charles D Gomersall 1,✉, Gavin M Joynt 1
To date H5N1 avian influenza viruses have infected 165 persons and caused 88 deaths in several Asian and one European country (http://www.who.int/csr/disease/avian_influenza/country/cases_table_2006_02_06/en/index.html; accessed 6 February 2006).
17. 'The Autumn Ghost' tells of innovation that saved lives during polio epidemic, September 18, 2023. https://www.wbur.org/hereandnow/2023/09/18/the-autumn-ghost-polio
“The iron lung, which came in in 1928, was really revolutionary,” says critical care physician Dr. Hannah Wunsch. “In fact, it was the first time you had a machine that could support people's breathing.”
Though revolutionary, it still had a host of setbacks. Patients often felt trapped in a huge metal casket, a major downside to the iron lung. Plus, doctors and nurses had trouble treating patients because they had to stay encapsulated in the machine to create negative pressure that would suck air into the lungs. Additionally, for patients with bulbar polio, the mortality rate still hovered around 90% even when using the iron lung.
To address these shortcomings, Dr. Bjørn Aage Ibsen started working to create a positive pressure ventilator in 1953. The technology would effectively push air into the lungs. And, the technology was much smaller and less invasive than the iron lung. The mortality rate of the disease reduced from 90% to 20%
18. Clinical Features of Nipah Virus Encephalitis among Pig Farmers in Malaysia, Goh et al, April 27, 2000. https://www.nejm.org/doi/full/10.1056/NEJM200004273421701
19. A Cohort Study of Health Care Workers to Assess Nosocomial Transmissibility of Nipah Virus, Malaysia, 1999. Mounts et al, https://www.researchgate.net/publication/12150124_A_Cohort_Study_of_Health_Care_Workers_to_Assess_Nosocomial_Transmissibility_of_Nipah_Virus_Malaysia_1999
20. A major outbreak of severe acute respiratory syndrome in Hong Kong, Lee et al, May 15, 2003. https://pubmed.ncbi.nlm.nih.gov/12682352/
21. Severe acute respiratory syndrome: review and lessons of the 2003 outbreak. Parasher and Anderson, September 2004. https://pmc.ncbi.nlm.nih.gov/articles/PMC7108628/
22. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Yang et al, February 26, 2020. https://pmc.ncbi.nlm.nih.gov/articles/PMC7102538/
23. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study, Ziehr et al, June 15, 2020. https://www.atsjournals.org/doi/full/10.1164/rccm.202004-1163LE
24. Invasive mechanical ventilation in COVID-19 patient management: the experience with 469 patients in Wuhan, Hua et al, June 16, 2020. https://pmc.ncbi.nlm.nih.gov/articles/PMC7296285/
25. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, Richardson et al, April 22, 2020. https://jamanetwork.com/journals/jama/fullarticle/2765184
26. Dr. Pierre Kory testimony to Senate Committee on Homeland Security and Governmental Affairs, December 8, 2020. https://rumble.com/v68pjmg-dr.-pierre-kory-says-he-watched-ventilated-sedated-and-paralyzed-patients-d.html
I'm a lung specialist! I'm an ICU specialist! I've cared from more dying COVID patients than anyone can imagine. They're dying because they can't breathe. They can't breathe. They're on high flow oxygen delivery devices. They're on noninvasive ventilators and/or they're sedated and paralyzed and attached to mechanical ventilators that breathe for them. And I watch them every day. They die.
END










What test is there for bird flu? What methodology? What swab do they jab up the chicken’s nose? Since airborne viruses die in sunlight and fresh air, how do birds infect each other? Are other birds getting the flu?
Extremely well written; substantiated with in-depth research. Wealth of information indeed!